-
ClearPoint Neuro Announces FDA Clearance for Software Version 2.1
ソース: Nasdaq GlobeNewswire / 19 9 2022 16:13:08 America/New_York
SOLANA BEACH, Calif., Sept. 19, 2022 (GLOBE NEWSWIRE) -- ClearPoint Neuro, Inc. (Nasdaq: CLPT) (the “Company”), a global therapy-enabling platform company providing navigation and delivery to the brain, today announced that it has obtained 510(k) clearance for version 2.1 of the ClearPoint Neuro Navigation software.
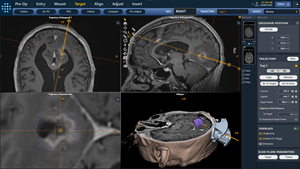
Version 2.1 of the ClearPoint System is intended to provide stereotactic guidance for the placement and operation of instruments or devices during planning and operation of neurological procedures within the MRI environment and in conjunction with MR imaging. The ClearPoint System is intended as an integral part of procedures that have traditionally used stereotactic methodology. These procedures include biopsies, catheter and electrode insertion, including deep brain stimulation (DBS) lead placement. The System is intended for use only with 1.5 and 3.0 Tesla MRI scanners.
The main customer benefits of the 2.1 software include optimizing ease of use for clinicians, enhancing visualization of medical image datasets, providing a new set of trajectory planning tools, introducing new workflow tools for gene therapy clinical trials, and numerous performance and technical improvements which will help to streamline and optimize the clinical workflow. The software is currently in limited market release and will be deployed initially to ClearPoint customers who participate in the ClearPoint “Pathfinder” Program. Pathfinder is designed to support and cultivate first-hand discovery of innovative developments that may facilitate optimal patient care by providing participating customer sites with access to ClearPoint Neuro’s cutting-edge technology.
“The release of the ClearPoint 2.1 Software is a fantastic achievement for the company and provides our customer base with a significant set of software-related improvements which will enhance the usability of the product and optimize the clinical workflow,” commented Tim Orr, Vice President of Software Engineering at ClearPoint Neuro. “In addition to offering new image visualization capabilities and performance improvements, this version offers a rich set of functionalities which will strengthen an already comprehensive set of trajectory planning and guidance tools within the software. Over the years of developing an image-guided platform for neurosurgical procedures, we have paid very close attention to the differing workflows that clinicians have used with our system and have incorporated several important features in this version which we believe will better optimize their intraoperative clinical workflows. More importantly, this version has introduced numerous functional building blocks which we hope will lay the groundwork for significant future growth in the areas of preoperative planning, medical image visualization, and automatic image fusion. Our goal with this release is to continue to offer our customers a world-class image-guided planning and navigation software system that they feel confident using for preoperative planning and intraoperative guidance.”
The Principal Software Architect for ClearPoint Neuro, Phil Hotte commented: “This latest release builds on the tremendous success of the ClearPoint software, including many new features and improvements suggested by our users while also setting the stage for the next generations of ClearPoint products. This is a great achievement for us, but this is still just the beginning. Our path forward will take full advantage of the capabilities of the recently cleared ClearPoint Maestro™ Brain Model, giving us a new engine to drive further innovation in all our products.”
About ClearPoint Neuro
ClearPoint Neuro’s mission is to improve and restore quality of life to patients and their families by enabling therapies for the most complex neurological disorders with pinpoint accuracy. Applications of the Company’s current product portfolio include deep brain stimulation, laser ablation, biopsy, neuro-aspiration, and delivery of drugs, biologics, and gene therapy to the brain. The ClearPoint® Neuro Navigation System has FDA clearance, is CE-marked, and is installed in over 60 active sites in the United States, Canada, and Europe. ClearPoint Neuro is partnered with more than 45 pharmaceutical and biotech companies, academic institutions, and contract research organizations providing solutions for direct CNS delivery of therapeutics in pre-clinical studies and clinical trials worldwide. To date, more than 5,000 cases have been performed and supported by the Company’s field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
Statements in this press release concerning the Company’s plans, growth and strategies may include forward-looking statements within the context of the federal securities laws. Statements regarding the Company's future events, developments and future performance, as well as management's expectations, beliefs, plans, estimates or projections relating to the future, are forward-looking statements within the meaning of these laws. Uncertainties and risks may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: the impact of the COVID-19 pandemic and global instability, supply chain disruptions, labor shortages, and inflationary conditions; future revenue from sales of the Company’s ClearPoint Neuro Navigation System and other new products offered by the Company; the Company’s ability to market, commercialize and achieve broader market acceptance for the Company’s ClearPoint Neuro Navigation System and other new products offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of our products and services in their delivery of therapies; and risks inherent in the research and development of new products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2021, and the Company’s Quarterly Report on Form 10-Q for the three months ended June 30, 2022, both of which have been filed with the Securities and Exchange Commission.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/664c63d7-c76a-48a2-8011-fc0ff677157e

Contact: Jacqueline Keller, Vice President, Marketing 1 (888) 287-9109 info@clearpointneuro.com Caroline Corner, Investor Relations ir@clearpointneuro.com